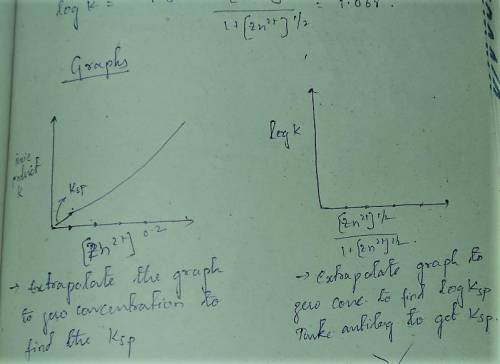
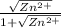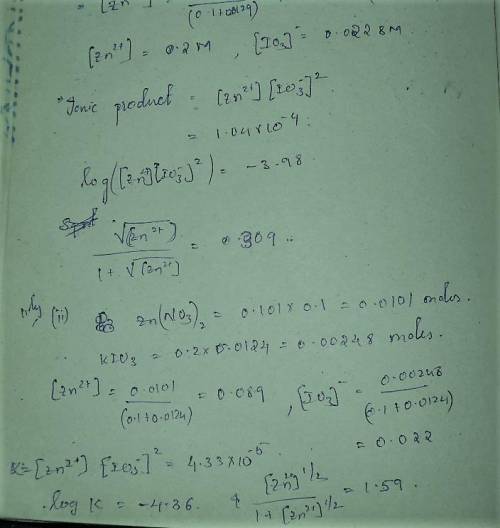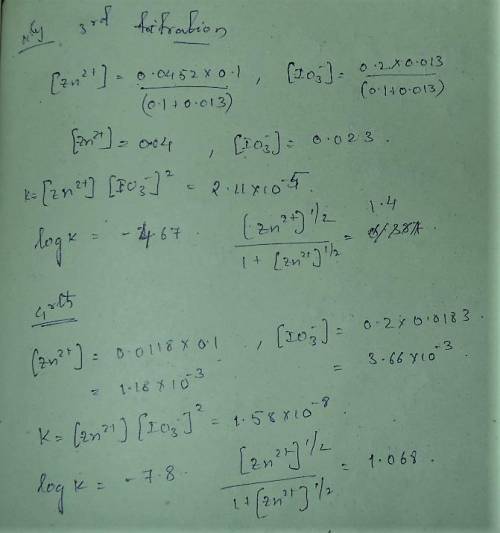A student followed the procedure of this experiment to determine the solubility product of zinc(II) iodate, Zn(IO3)2. Solutions of ZN(NO3)2 of known initial concentrations were titrated with 0.200 M KIO3 solutions to the first appearance of a white precipitate. The following data were collected.
Complete the table below and determine the solubility product constant.
[Zn(NO3)2]0, M Initial 0.226, 0.101 0.0452, 0.0118
[KIO3]0, M Titrant 0.200, 0.200, 0.200, 0.200
V0, mL of Zn(NO3)2 100.0, 100.0, 100.0, 100.0
V, mL of KIO3 titrant 12.9, 12.4, 13.0, 18.3

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
Do you know the correct answer?
A student followed the procedure of this experiment to determine the solubility product of zinc(II)...
Questions in other subjects:

English, 27.10.2020 20:00

History, 27.10.2020 20:00

Physics, 27.10.2020 20:00

Arts, 27.10.2020 20:00



Chemistry, 27.10.2020 20:00


Chemistry, 27.10.2020 20:00

Mathematics, 27.10.2020 20:00