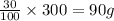Chemistry, 20.04.2020 20:47, Alienhead6187
An ionic compound has a solubility of 30 g per 100 mL of water at room temperature. A solution containing 70 g of the compound in 300 mL of water at the same temperature is:
A. unsaturated.
B. saturated.
C. a suspension.
D. supersaturated.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 23.06.2019 15:00, ctyrector
20 look at the clock and the data table below. based on the data and on your knowledge of potential and kinetic energy, what is the best conclusion you can make about potential and kinetic energy? the total amount of energy stays the same. the clock has the most potential energy at point b since it is moving the fastest. there is always more potential energy than kinetic energy. potential energy can never be 0, but you can have 0 kinetic energy.
Answers: 1

Do you know the correct answer?
An ionic compound has a solubility of 30 g per 100 mL of water at room temperature. A solution conta...
Questions in other subjects:

Mathematics, 06.01.2020 19:31


Geography, 06.01.2020 19:31

Mathematics, 06.01.2020 19:31




Mathematics, 06.01.2020 19:31


Biology, 06.01.2020 19:31