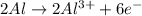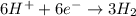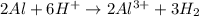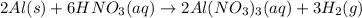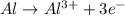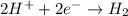I'm the redox reaction of sodium aluminum metal reacts with nitric acid it will form products of aluminum nitrate and molecular hydrogen. Write the balanced half reactions and identify each one as either oxidation or reduction. Find the final net balanced reaction from the half reactions.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1


Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
Do you know the correct answer?
I'm the redox reaction of sodium aluminum metal reacts with nitric acid it will form products of alu...
Questions in other subjects:

History, 08.12.2020 18:30

Mathematics, 08.12.2020 18:30


World Languages, 08.12.2020 18:30

Physics, 08.12.2020 18:30



Mathematics, 08.12.2020 18:30

Mathematics, 08.12.2020 18:30