
Chemistry, 20.04.2020 14:30, jflandersneongr
Aluminum Oxide is formed when aluminum combines with oxygen in the air. How many grams of Al2O3 are formed when 23.6g of Al reacts completely with oxygen?
4 Al + 3 O2 —> 2 Al2O3
A. 44.6 grams B. 35.6 grams C. 87.6 grams D. 21.6 grams

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1


Chemistry, 22.06.2019 23:30, ninilizovtskt
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
Do you know the correct answer?
Aluminum Oxide is formed when aluminum combines with oxygen in the air. How many grams of Al2O3 are...
Questions in other subjects:




Mathematics, 17.05.2021 01:20


Mathematics, 17.05.2021 01:20



Mathematics, 17.05.2021 01:20

Social Studies, 17.05.2021 01:20


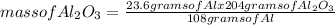
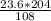 = 44.6 g of Al₂O₃
= 44.6 g of Al₂O₃ 



