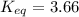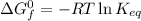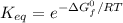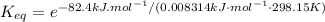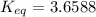Calculate the value of the equilibrium constant (K) at 25 °C for the reaction
2 NOBr(g) = N2(g)...

Chemistry, 20.04.2020 03:26, officialkk
Calculate the value of the equilibrium constant (K) at 25 °C for the reaction
2 NOBr(g) = N2(g) + O2(g) + Br2()
given that the standard free energy of formation (AG°f) of NOBr(g) is 82.4 kJ/mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, TheRealKodakBlack
What is a scientific theory? a. a scientist's guess about how something works b. the results of an experiment obtained using the scientific method c. a proven fact that will never change d. an idea that is backed by data from many sources
Answers: 2

Chemistry, 21.06.2019 16:30, girlwholikesanime
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 21.06.2019 18:30, brookekolmetz
How many orbitals does the p sub shell container
Answers: 3

Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Geography, 23.06.2019 23:30

History, 23.06.2019 23:30





Mathematics, 23.06.2019 23:30



History, 23.06.2019 23:30