
Chemistry, 20.04.2020 03:25, amakayla57
At equilibrium, a 1.0 L reaction vessel contains 2.3 mol of Mg(OH)₂ and 0.170 mol of OH⁻. What is the equilibrium concentration of Mg²⁺ based on the reaction:
Mg(OH)₂ (s) ⇌ Mg²⁺ (aq) + 2 OH⁻ (aq) Kc = 1.80 x 10⁻¹¹

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
Do you know the correct answer?
At equilibrium, a 1.0 L reaction vessel contains 2.3 mol of Mg(OH)₂ and 0.170 mol of OH⁻. What is th...
Questions in other subjects:

Chemistry, 11.10.2019 16:50


Social Studies, 11.10.2019 16:50




Mathematics, 11.10.2019 16:50

Physics, 11.10.2019 16:50



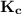 relates to the summation of the initial concentration and the change gotten from its stoichiometric reaction.
relates to the summation of the initial concentration and the change gotten from its stoichiometric reaction. ![\mathbf{K_c = \dfrac{[Mg^{2+}_{(aq)}] [OH^-]^2}{[Mg(OH)_2_{(s)}]} }](/tpl/images/0611/5877/2058a.png)
![\mathbf{1.8 \times 10^{-11} = \dfrac{[Mg^{2+}_{(aq)}] (0.170)^2}{1} }](/tpl/images/0611/5877/d71f0.png)
![\mathbf{ [Mg^{2+}_{(aq)}] = \dfrac{ 1 .8 \times 10^{-11} }{(0.170)^2} }](/tpl/images/0611/5877/a67b7.png)
![\mathbf{ [Mg^{2+}_{(aq)}] =6.22 \times 10^{-10}}](/tpl/images/0611/5877/88f0d.png)
![\mathbf{ [Mg^{2+}_{(aq)}] =0.622 \times 10^{-9}}](/tpl/images/0611/5877/452fc.png)





