
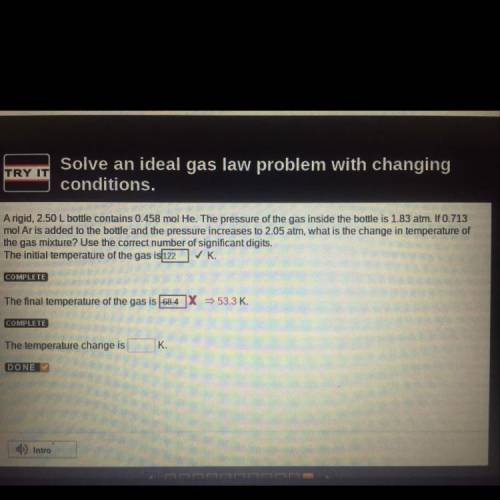
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm. If 0.713
mol Ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of
the gas mixture? Use the correct number of significant digits.
The temperature change is

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
Do you know the correct answer?
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm....
Questions in other subjects:


History, 17.12.2019 15:31

Mathematics, 17.12.2019 15:31


Biology, 17.12.2019 15:31


Health, 17.12.2019 15:31


Biology, 17.12.2019 15:31

Biology, 17.12.2019 15:31






