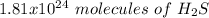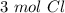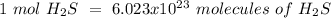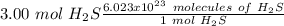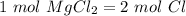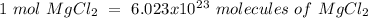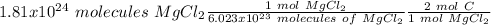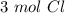Chemistry, 14.11.2019 10:31, MadisonUpky9652
Using avogadro's number (6.02*10^23): calculate the number of molecules in 3.00 moles h2s . express your answer numerically in molecules.. calculate the number of moles of cl atoms in 1.81×10^24 formula units of magnesium chloride, mgcl2 .

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 06:30, reecedstceklein
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 23:30, adamgala3885
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
Do you know the correct answer?
Using avogadro's number (6.02*10^23): calculate the number of molecules in 3.00 moles h2s . express...
Questions in other subjects:

Mathematics, 28.01.2021 01:20

SAT, 28.01.2021 01:20

Mathematics, 28.01.2021 01:20

Social Studies, 28.01.2021 01:20


Physics, 28.01.2021 01:20


Mathematics, 28.01.2021 01:20


Biology, 28.01.2021 01:20