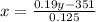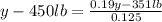Chemistry, 18.04.2020 04:51, mansoorahmedd67
(Eide Problem 14.20) Dilute sulfuric acid (19% acid and the rest water) is required for activating car batteries. A tank of weak acid (12.5% acid and the rest water) is available. If 450 lbs of 78% concentrate acid is added to the tank to get the required 19% acid, how much of the 19% acid is now available?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
Do you know the correct answer?
(Eide Problem 14.20) Dilute sulfuric acid (19% acid and the rest water) is required for activating c...
Questions in other subjects:


Mathematics, 20.08.2021 17:40