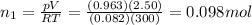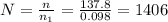Chemistry, 17.04.2020 22:16, gizmo50245
You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 ∞C. The He is used to fill balloons to a volume of 2.50 L at 732 mm Hg and 27 ∞C. How many balloons can be filled with He? Assume that the cylinder can provide He until its internal pressure reaches 1.00 atm (i. e., there are 131 atmospheres of usable He in the cylinder).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 22.06.2019 21:00, thebasedgodchri
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 00:00, juliannasl
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
Do you know the correct answer?
You have a 25.0 L cylinder of helium at a pressure of 132 atm and a temperature of 19 ∞C. The He is...
Questions in other subjects:


World Languages, 02.03.2021 22:50

Mathematics, 02.03.2021 22:50


English, 02.03.2021 22:50


Mathematics, 02.03.2021 22:50

Mathematics, 02.03.2021 22:50



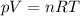
 is the gas constant
is the gas constant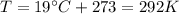 is the temperature
is the temperature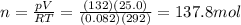
 is the pressure
is the pressure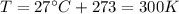 is the temperature
is the temperature