
Chemistry, 02.10.2019 12:00, janahiac09
An equilibrium mixture of co, o2 and co2 at a certain temperature contains 0.0010 m co2 and 0.0100 m o2. at this temperature, kc equals 1.4 × 102 for the reaction: 2 co(g) + o2(g)⇌2 co2(g). what is the equilibrium concentration of co?
a) 7.1 × 10-7 m.
b) 8.4 × 10-4 m.
c) 1.4 × 10-2 m.
d) 1.2 × 10-1 m.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3


Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1
Do you know the correct answer?
An equilibrium mixture of co, o2 and co2 at a certain temperature contains 0.0010 m co2 and 0.0100 m...
Questions in other subjects:



Chemistry, 18.07.2019 10:30

Mathematics, 18.07.2019 10:30

Mathematics, 18.07.2019 10:30



Mathematics, 18.07.2019 10:30


Physics, 18.07.2019 10:30


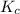
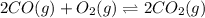
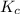 for above reaction is:
for above reaction is:![K_c=\frac{[CO_2]^2}{[CO]^2[O_2]}](/tpl/images/0282/9569/3f046.png)
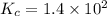
![[CO_2]=0.0010M](/tpl/images/0282/9569/bba46.png)
![[O_2]=0.0100M](/tpl/images/0282/9569/e6846.png)
![1.4\times 10^2=\frac{(0.0010)^2}{[CO]^2\times (0.0100)}](/tpl/images/0282/9569/4f227.png)
![[CO]=\sqrt{\frac{(0.0010)^2}{0.0100\times 1.4\times 10^2}}](/tpl/images/0282/9569/54368.png)
![[CO]=8.4\times 10^{-4}M](/tpl/images/0282/9569/712c0.png)




