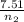A flexible container at an initial volume of 8.15 L contains 7.51 mol of a gas. More gas is
th...

Chemistry, 16.04.2020 23:03, Arianaagosto
A flexible container at an initial volume of 8.15 L contains 7.51 mol of a gas. More gas is
then added to the container until it reaches a final volume of 13.9 L. Assuming the
pressure and the temperature of the gas remained constant, calculate the moles of gas
added to the container.
15.1 mol
O 5.5 mol
0 4.40 mol
12.8 mol

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2


Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 28.10.2020 03:30

Mathematics, 28.10.2020 03:30




History, 28.10.2020 03:30

Mathematics, 28.10.2020 03:30


Mathematics, 28.10.2020 03:30


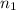 ) = 7.51 moles
) = 7.51 moles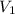 ) = 8.15 L
) = 8.15 L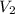 ) = 13.9 L
) = 13.9 L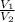 =
= 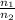
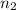 represents the final moles
represents the final moles 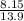 =
=