
Chemistry, 16.04.2020 20:01, Jerryholloway5871
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
2NaN3(s)-> 2Na(s) + 3N2(s)
An unknown amount of sodium azide (NaN3) reacts and produces 744 mL of nitrogen gas. What mass of sodium metal is produced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, keananashville
Metallic bonds are good conductors of electricity true or false
Answers: 2

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
Do you know the correct answer?
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
...
...
Questions in other subjects:




Mathematics, 26.03.2020 22:54

History, 26.03.2020 22:54

English, 26.03.2020 22:54


Mathematics, 26.03.2020 22:54


Mathematics, 26.03.2020 22:54


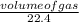
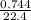 = 0.03moles
= 0.03moles 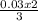 = 0.02mole
= 0.02mole



