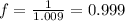Chemistry, 16.04.2020 04:57, pleasedontspamme
You prepare a buffer solution from 10.0 mL of 0.100 M MOPS (3‑morpholinopropane‑1‑sulfonic acid) and 10.0 mL of 0.074 M NaOH . 0.074 M NaOH. Next, you add 1.00 mL of 3.51 × 10 − 5 M 3.51×10−5 M lidocaine to this mixture. Denoting lidocaine as L, calculate the fraction of lidocaine present in the form LH +

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
Do you know the correct answer?
You prepare a buffer solution from 10.0 mL of 0.100 M MOPS (3‑morpholinopropane‑1‑sulfonic acid) and...
Questions in other subjects:

Physics, 14.05.2021 03:10

Mathematics, 14.05.2021 03:10



Mathematics, 14.05.2021 03:10



Physics, 14.05.2021 03:10

English, 14.05.2021 03:10


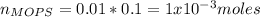
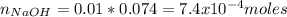
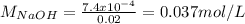
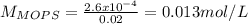
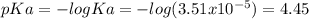
![pH=pKa+log\frac{[NaOH]}{[MOPS]} =4.45+log\frac{0.037}{0.013} =4.9](/tpl/images/0604/7273/c3441.png)
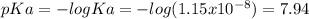
![pH=pKa+log\frac{[base]}{[acid]} \\4.9=7.94+log\frac{[base]}{[acid]}\\log\frac{[base]}{[acid]}=-3.04\\base/acid=9.12x10^{-4}](/tpl/images/0604/7273/6eb33.png)