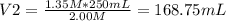You need to prepare 250.0 mL of a 1.35 M HCl solution from a 2.00 M HCl stock solution.
...

Chemistry, 16.04.2020 04:56, Bjehnsen3720
You need to prepare 250.0 mL of a 1.35 M HCl solution from a 2.00 M HCl stock solution.
a. Which glassware should you use to make the solution?
A. beaker
B. Erlenmeyer flask
C. volumetric flask
b. How should the correct amount of stock be obtained?
A. Measure out x g on a balance
B. Measure out x mL using a volumetric pipet
C. Measure out x mL using a graduated cylinder
c. Based on your answer above, what is the value of x?
d. How should the solution be mixed together?
A. Fill the container to the 250 mL mark then add the correct amount of stock solution.
B. Add the correct amount of stock solution then fill to the 250 mL mark with water.
C. Fill the container partially with water, add the correct amount of stock solution, then fill to the 250 mL mark with water.
D. None of these is the correct way to mix the stock solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, alevans7144
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1
Do you know the correct answer?
Questions in other subjects:






Mathematics, 11.10.2020 06:01


Mathematics, 11.10.2020 06:01

Mathematics, 11.10.2020 06:01