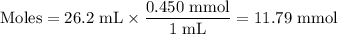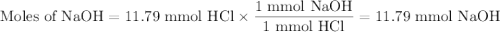Chemistry, 16.04.2020 03:18, Kingmoney959
26.2 mL of a 0.450 M hydrochloric acid solution is titrated with an unknown concentration of sodium hydroxide. 45.8 mL of the sodium hydroxide solution is required to reach the equivalence point. What is the molar concentration of the sodium hydroxide solution?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1


Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
Do you know the correct answer?
26.2 mL of a 0.450 M hydrochloric acid solution is titrated with an unknown concentration of sodium...
Questions in other subjects:

Mathematics, 26.11.2020 07:50


Biology, 26.11.2020 07:50

Biology, 26.11.2020 07:50

Arts, 26.11.2020 07:50





English, 26.11.2020 08:00