Chemistry, 16.04.2020 02:31, hebrew1148
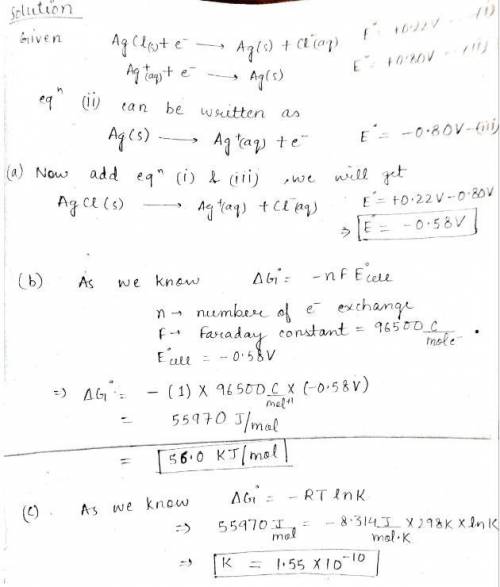
You are given the following half-reactions. AgCl(s) + e− → Ag(s) + Cl −(aq) ℰ° = +0.22 V Ag+(aq) + e− → Ag(s) ℰ° = +0.80 V Construct a cell with the following cell reaction. AgCl(s) → Ag+(aq) + Cl −(aq) Please see Adding Reactions for assistance. (a) What is the standard cell potential? WebAssign will check your answer for the correct number of significant figures. -.58 Correct: Your answer is correct. V (b) What is the value of ΔG° for the reaction? WebAssign will check your answer for the correct number of significant figures. 56 Correct: Your answer is correct. kJ/mol (c) What is the equilibrium constant as determined from the cell potential? (Please recall that often a significant figures appears to be lost when raising 10 to a power.) WebAssign will check your answer for the correct number of significant figures. 1.6e-10 Incorrect: Your answer is incorrect.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2



Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
Do you know the correct answer?
You are given the following half-reactions. AgCl(s) + e− → Ag(s) + Cl −(aq) ℰ° = +0.22 V Ag+(aq) + e...
Questions in other subjects:

Mathematics, 23.03.2020 23:57



Mathematics, 23.03.2020 23:57

History, 23.03.2020 23:57


Mathematics, 23.03.2020 23:57

Mathematics, 23.03.2020 23:57