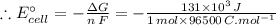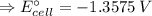Chemistry, 16.04.2020 02:02, kaylienguyen
The free energy change for the following reaction at 25 °C, when [Cr3+] = 1.32×10-3 M and [Fe3+] = 1.14 M, is 131 kJ: Cr3+(1.32×10-3 M) + Fe2+(aq) Cr2+(aq) + Fe3+(1.14 M) ΔG = 131 kJ What is the cell potential for the reaction as written under these conditions? V Would this reaction be spontaneous in the forward or the reverse direction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 04:20, tatta95
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1

Chemistry, 23.06.2019 05:30, jalynholden07
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 11:20, emmaraeschool
Match each state of matter with the statement that best describes it.
Answers: 1
Do you know the correct answer?
The free energy change for the following reaction at 25 °C, when [Cr3+] = 1.32×10-3 M and [Fe3+] = 1...
Questions in other subjects:


Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10






Mathematics, 18.03.2021 01:10