
The standard enthalpy change for the combustion of 1 mole of propane is –2043.0 kJ. C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g) Calculate ΔfH° for propane based on the following standard molar enthalpies of formation. molecule ΔfH° (kJ/mol-rxn) CO2(g) –393.5 H2O(g) –241.8'

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
Do you know the correct answer?
The standard enthalpy change for the combustion of 1 mole of propane is –2043.0 kJ. C3H8(g) + 5 O2(g...
Questions in other subjects:





Chemistry, 27.01.2020 10:31


History, 27.01.2020 10:31


Biology, 27.01.2020 10:31

Computers and Technology, 27.01.2020 10:31


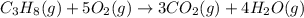
![\Delta H^o_{rxn}=[(3\times \Delta H^o_f_{(CO_2(g))})+(4\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0604/1723/6eb42.png)
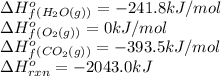
![-2043.0=[(3\times (-393.5))+(4\times (-241.8))]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times (0))]\\\\\Delta H^o_f_{(C_3H_8(g))}=-104.7kJ/mol](/tpl/images/0604/1723/1b82f.png)
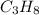 is -104.7 kJ/mol
is -104.7 kJ/mol



