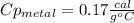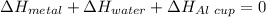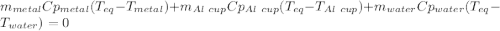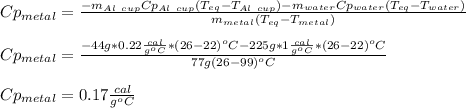Chemistry, 16.04.2020 01:02, isabelperez063
77 grams of an unknown metal at 99ᵒC is placed in 225 grams of water which is initially at 22ᵒC. The water is inside a 44 gram aluminum cup with a specific heat of 0.22 cal/gᵒC. The final temperature of the system is 26ᵒC. What is the specific heat of the unknown metal?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 01:00, crysderria
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
77 grams of an unknown metal at 99ᵒC is placed in 225 grams of water which is initially at 22ᵒC. The...
Questions in other subjects:



History, 09.07.2019 08:50

History, 09.07.2019 08:50

Chemistry, 09.07.2019 08:50


History, 09.07.2019 08:50


Biology, 09.07.2019 08:50