

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 07:30, isalih7256
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1

Chemistry, 23.06.2019 09:00, blossie94681
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1

Chemistry, 23.06.2019 10:30, dreamxette3119
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
Do you know the correct answer?
An ideal gas originally at 0.85 atm and 66°C was allowed to expand until its final volume, pressure...
Questions in other subjects:

Mathematics, 24.04.2020 22:54


Mathematics, 24.04.2020 22:54

English, 24.04.2020 22:54



Mathematics, 24.04.2020 22:54

English, 24.04.2020 22:54




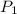 = initial pressure of gas = 0.85 atm
= initial pressure of gas = 0.85 atm = final pressure of gas = 456 mm Hg = 0.60 atm (760mmHg=1atm)
= final pressure of gas = 456 mm Hg = 0.60 atm (760mmHg=1atm)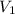 = initial volume of gas = ?
= initial volume of gas = ?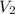 = final volume of gas = 94.0 ml
= final volume of gas = 94.0 ml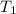 = initial temperature of gas =
= initial temperature of gas = 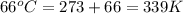
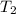 = final temperature of gas =
= final temperature of gas = 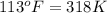
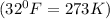
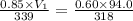
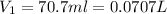 (1L=1000ml)
(1L=1000ml)




