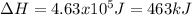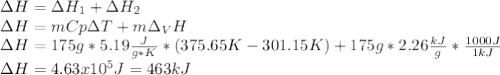Salt water can be desalinated by distillation. How much energy is needed to convert 175 g of salt water at 28.0 °C to water vapor if the specific heat of salt water is 5.19 J/g K, the boiling point of salt water is 102.5 °C, and the enthalpy of vaporization is 2.26 kJ/g?
A. 317 kJ
B. 399 kJ
C. 463 kJ
D. 512 kJ
E. 673 kJ

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 23:30, lizdeleon248
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1

Do you know the correct answer?
Salt water can be desalinated by distillation. How much energy is needed to convert 175 g of salt wa...
Questions in other subjects:


Mathematics, 12.08.2021 14:00

Physics, 12.08.2021 14:00

Mathematics, 12.08.2021 14:00


English, 12.08.2021 14:00

English, 12.08.2021 14:00


Social Studies, 12.08.2021 14:00

English, 12.08.2021 14:00