Chemistry, 16.04.2020 00:23, lerasteidl
Measurements show that the enthalpy of a mixture of gaseous reactants increases by 243.kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that −174.kJ of work is done on the mixture during the reaction. Calculate the change of energy of the gas mixture during the reaction in kJ.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 23.06.2019 04:00, anonymous1813
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Do you know the correct answer?
Measurements show that the enthalpy of a mixture of gaseous reactants increases by 243.kJ during a c...
Questions in other subjects:


Mathematics, 04.03.2020 00:30

Computers and Technology, 04.03.2020 00:30

Physics, 04.03.2020 00:30







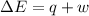
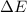 =Change in internal energy
=Change in internal energy