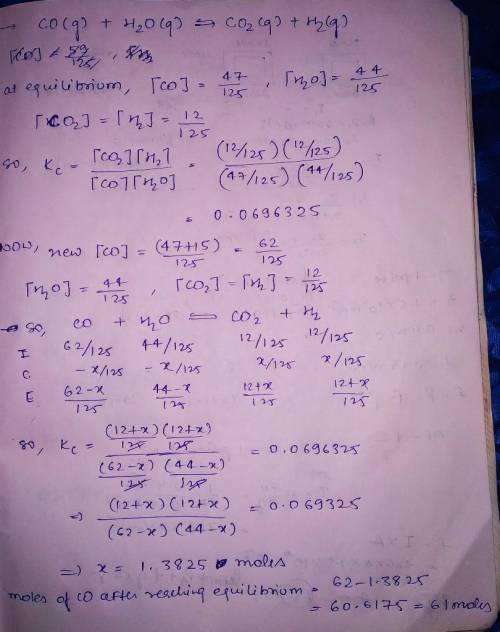Chemistry, 15.04.2020 23:28, mathscience9301
"Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas will form carbon dioxide and hydrogen, and in fact this reaction is one of the ways hydrogen is made industrially. A chemical engineer studying this reaction fills a tank with of carbon monoxide gas and of water vapor. When the mixture has come to equilibrium he determines that it contains of carbon monoxide gas, of water vapor and of carbon dioxide. The engineer then adds another of carbon monoxide, and allows the mixture to come to equilibrium again. Calculate the moles of after equilibrium is reached the second time. Round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
Do you know the correct answer?
"Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas w...
Questions in other subjects:

Mathematics, 13.12.2020 01:00



Mathematics, 13.12.2020 01:00


Mathematics, 13.12.2020 01:00

Biology, 13.12.2020 01:00


Mathematics, 13.12.2020 01:00

Social Studies, 13.12.2020 01:00