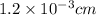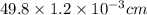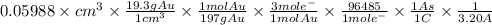Chemistry, 15.04.2020 23:16, lilsnsbsbs
The surface area of an object to be gold plated is 49.8 cm2 and the density of gold is 19.3 g/cm-3. A current of 3.15. A is applied to a solution that contains gold in the +3 oxidation state. Calculate the time required to deposit an even layer of gold 1.2 x 10 -3cm thick on the object.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1


Chemistry, 22.06.2019 23:00, lilsnsbsbs
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
Do you know the correct answer?
The surface area of an object to be gold plated is 49.8 cm2 and the density of gold is 19.3 g/cm-3....
Questions in other subjects:


Spanish, 04.09.2019 20:30


History, 04.09.2019 20:30

History, 04.09.2019 20:30


History, 04.09.2019 20:30

Mathematics, 04.09.2019 20:30

History, 04.09.2019 20:30


 sec.
sec.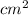 ,
,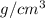 ,
,