
You have a solution of two volatile liquids, A and B (assume ideal behavior). Pure liquid A has a vapor pressure of 385.0 torr and pure liquid B has a vapor pressure of 104.0 torr at the temperature of the solution. The vapor at equilibrium above the solution has double the mole fraction of substance A as the solution does. What is the mole fraction of liquid A in the solution?

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 13:30, katswindle11
Asap at one point in history people could measure temperature by looking at the volume of a sample of gas. suppose a sample in a gas thermometer has a volume of 135ml at 11.0°c. indicate what temperature would correspond to each of the following volumes: 113 ml, and 155 ml.
Answers: 1
Do you know the correct answer?
You have a solution of two volatile liquids, A and B (assume ideal behavior). Pure liquid A has a va...
Questions in other subjects:

Mathematics, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40


Mathematics, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40

Mathematics, 24.03.2021 17:40




English, 24.03.2021 17:40


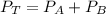 ---------- Equation (1)
---------- Equation (1)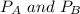 are partial pressure of A and B respectively.
are partial pressure of A and B respectively.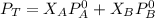 ---------- Equation (2)
---------- Equation (2)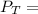 the total vapor pressure of the solution
the total vapor pressure of the solution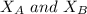 = mole fraction of A and B respectively
= mole fraction of A and B respectively 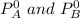 = vapor pressures of pure species of A and B
= vapor pressures of pure species of A and B 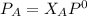
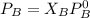
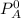 and
and 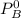 into equation (2) ; we have:
into equation (2) ; we have: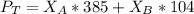
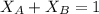
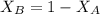

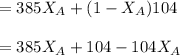
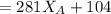 ------ Equation (3)
------ Equation (3)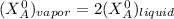
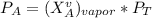
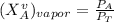
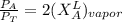 ------- Equation (4)
------- Equation (4)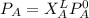
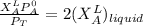
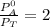
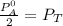
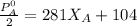
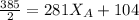

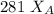
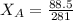
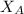 = 0.32
= 0.32 



