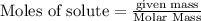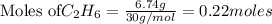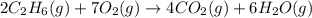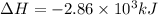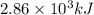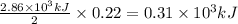Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1

Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 01:00, only1cache
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
Do you know the correct answer?
The following thermochemical equation is for the reaction of ethane(g) with oxygen(g) to form carbon...
Questions in other subjects:




Mathematics, 09.07.2020 14:01

History, 09.07.2020 14:01

History, 09.07.2020 14:01


Mathematics, 09.07.2020 14:01

Health, 09.07.2020 14:01


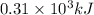 of energy is produced
of energy is produced