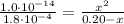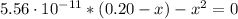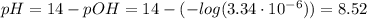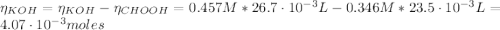Chemistry, 16.04.2020 00:36, RoxanneDuartee
Determine the pH during the titration of 23.5 mL of 0.346 M formic acid (Ka = 1.8×10-4) by 0.457 M KOH at the following points.
(a) Before the addition of any KOH
(b) After the addition of 4.00 mL of KOH
(c) At the half-equivalence point (the titration midpoint)
(d) At the equivalence point
(e) After the addition of 26.7 mL of KOH

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Do you know the correct answer?
Determine the pH during the titration of 23.5 mL of 0.346 M formic acid (Ka = 1.8×10-4) by 0.457 M K...
Questions in other subjects:


English, 22.10.2020 04:01




History, 22.10.2020 04:01


Biology, 22.10.2020 04:01


Mathematics, 22.10.2020 04:01


![pH = -log([H_{3}O^{+}])](/tpl/images/0603/8843/6ab72.png) (1)
(1) ![K_{a} = \frac{[CHOO^{-}][H_{3}O^{+}]}{[CHOOH]}](/tpl/images/0603/8843/c4333.png) (3)
(3)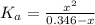
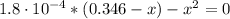 (4)
(4)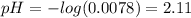
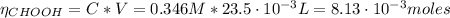
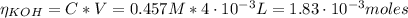
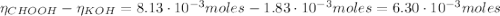
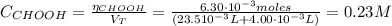
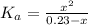
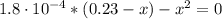
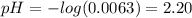
![pH = pKa + log(\frac{[CHOO^{-}]}{[CHOOH]})](/tpl/images/0603/8843/112dd.png)
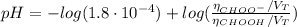
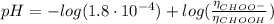
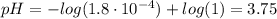
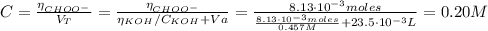
![K_{b} = \frac{[CHOOH][OH^{-}]}{[CHOO^{-}]}](/tpl/images/0603/8843/a45f9.png)
![\frac{K_{w}}{K_{a}} = \frac{[CHOOH][OH^{-}]}{[CHOO^{-}]}](/tpl/images/0603/8843/7611b.png)