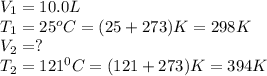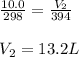Chemistry, 15.04.2020 20:57, blakesmith0110
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 629 torr. Using Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased 121 °C while maintaining the pressure at 629 torr.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 23.06.2019 03:30, alecnewman2002
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
Do you know the correct answer?
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 629 torr. Using Ch...
Questions in other subjects:






Social Studies, 03.10.2021 06:00

English, 03.10.2021 06:00



Mathematics, 03.10.2021 06:00


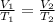
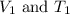 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.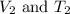 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.