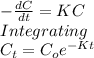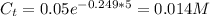Chemistry, 15.04.2020 20:40, EvoBruh4465
The breakdown of dopamine is catalyzed by the enzyme monoamine oxidase (MAO). What is the final concentration of product if the starting dopamine concentration is 0.050 M and the reaction runs for 5 seconds. (Assume the rate constant for the reaction is 0.249 s^-1.)
A) 0.050 M
B) 0.014 M
C) 0.018 M
D) 1.2 M
E) 0.025 M

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
Do you know the correct answer?
The breakdown of dopamine is catalyzed by the enzyme monoamine oxidase (MAO). What is the final conc...
Questions in other subjects:




English, 12.09.2019 05:30

SAT, 12.09.2019 05:30


Physics, 12.09.2019 05:30

Mathematics, 12.09.2019 05:30

History, 12.09.2019 05:30

Geography, 12.09.2019 05:30