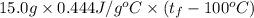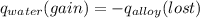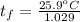Chemistry, 15.04.2020 20:48, jforeman42
A 15.0 g sample of nickel metal is heated to 100.0 degrees C and dropped into 55.0 g of water, initially at 23.0 degrees C. Assuming that all the heat lost by nickel is absorbed by the water, calculate the final temperature of the nickel and water. (C

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3



Do you know the correct answer?
A 15.0 g sample of nickel metal is heated to 100.0 degrees C and dropped into 55.0 g of water, initi...
Questions in other subjects:


Computers and Technology, 22.10.2020 18:01

Biology, 22.10.2020 18:01

Mathematics, 22.10.2020 18:01




Mathematics, 22.10.2020 18:01


Biology, 22.10.2020 18:01


 .
.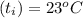 ,
, 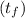 = ?,
= ?,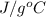 ,
, 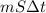
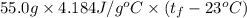
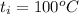 ,
,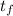 = ?
= ?