
Chemistry, 15.04.2020 18:26, FailingstudentXD
The reaction of Fe3O4(s) with hydrogen(g) to form iron(s) and water(g) proceeds as follows: Fe3O4(s) + 4 H2(g) 3 Fe(s) + 4 H2O(g) When 61.8 grams of Fe3O4(s) react with sufficient H2(g) , 40.3 kJ of energy are absorbed . What is the value of H for the chemical equation given?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1



Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
Do you know the correct answer?
The reaction of Fe3O4(s) with hydrogen(g) to form iron(s) and water(g) proceeds as follows: Fe3O4(s)...
Questions in other subjects:

Mathematics, 16.02.2021 07:30

Biology, 16.02.2021 07:30




Mathematics, 16.02.2021 07:30

Social Studies, 16.02.2021 07:30



Mathematics, 16.02.2021 07:30


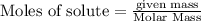
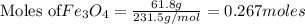
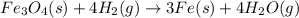
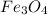 absorb energy = 40.3 kJ
absorb energy = 40.3 kJ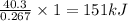
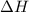 for the chemical equation given is 151 kJ
for the chemical equation given is 151 kJ



