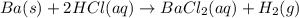Which of the following is an oxidation-reduction reaction? Which of the following is an oxidation-reduction reaction?
Pb(C2H3O2)2(aq) + 2 LiBr(aq) → PbBr2(s) + 2 LiC2H3O2(aq)
KI(aq) + AgNO3(aq) → AgI(s) + KNO3(aq)
HBr(aq) + KOH(aq) → KBr(aq) + H2O(l)
Ba(s) + 2 HCl(aq) → BaCl2(aq) + H2(g)
All of the above are oxidation-reduction reactions.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 19:30, toriabrocks
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2
Do you know the correct answer?
Which of the following is an oxidation-reduction reaction? Which of the following is an oxidation-re...
Questions in other subjects:


Spanish, 04.05.2021 23:30


Mathematics, 04.05.2021 23:30



Mathematics, 04.05.2021 23:30



English, 04.05.2021 23:30