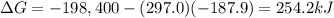For the process 2SO2(g) + O2(g) --> 2SO3(g),
ΔS = –187.9 J/K and ΔH = –198.4 kJ at 29...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, sophiapknight
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Biology, 06.11.2020 06:00

History, 06.11.2020 06:00

English, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00

Physics, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00

Mathematics, 06.11.2020 06:00




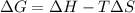
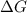 is the Gibbs free energy
is the Gibbs free energy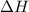 is the change in enthalpy of the reaction
is the change in enthalpy of the reaction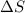 is the change in entropy
is the change in entropy