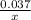Chemistry, 15.04.2020 04:48, anitaabbey27
Linolenic acid (C18H30O2) can be hydrogenated to stearic acid by reacting it with hydrogen gas according to the equation: C18H30O2 + 3H2 --->C18H36O2 What volume of hydrogen gas, measured at STP, is required to react with 10.5 g of linolenic acid in this reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, PineaPPle663
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 12:30, hayleyconsole
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3
Do you know the correct answer?
Linolenic acid (C18H30O2) can be hydrogenated to stearic acid by reacting it with hydrogen gas accor...
Questions in other subjects:



English, 09.02.2021 21:20


Chemistry, 09.02.2021 21:20

Mathematics, 09.02.2021 21:20

Mathematics, 09.02.2021 21:20

Mathematics, 09.02.2021 21:20


Mathematics, 09.02.2021 21:20


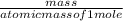
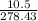
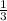 =
=