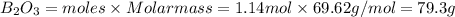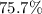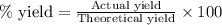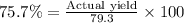Chemistry, 15.04.2020 03:45, danieldfuenteg732
If 36.9 mL of B2H6 reacted with excess oxygen gas, determine the actual yield of B2O3 if the percent yield of B2O3 was 75.7%. (The density of B2H6 is 1.131 g/mL. The molar mass of B2H6 is 27.668 g/mol and the molar mass of B2O3 is 69.62 g/mol.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Do you know the correct answer?
If 36.9 mL of B2H6 reacted with excess oxygen gas, determine the actual yield of B2O3 if the percent...
Questions in other subjects:



Mathematics, 15.06.2021 20:30


Mathematics, 15.06.2021 20:30



Biology, 15.06.2021 20:30

Mathematics, 15.06.2021 20:30


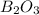 is 60.0 g
is 60.0 g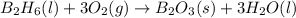
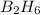 =
=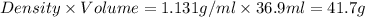
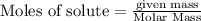
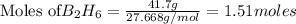
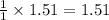 moles of
moles of