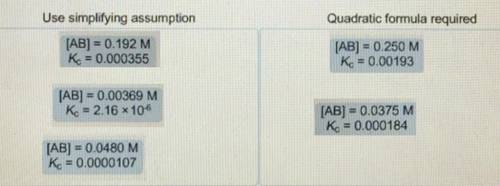
When determining the equilibrium concentrations for the reaction ; a simplifying assumption can be used under certain conditions to avoid solving a quadratic equation. Classify each situation by whether the simplifying assumption can be used or whether the quadratic formula is required. Use simplifying assumptions - Quadratic formula required 1. [AB] = 0.0178 M; 2. [AB] = 0.00204 M; 3. [AB] =0.451 M; = 0.000905 4. [AB] = 0.0174 M; = 0.0000925 5. [AB] = 0.396 M; = 0.00228

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:10, bossboybaker
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2
Do you know the correct answer?
When determining the equilibrium concentrations for the reaction ; a simplifying assumption can be...
Questions in other subjects:



History, 09.10.2019 06:50

Mathematics, 09.10.2019 06:50

Mathematics, 09.10.2019 06:50


Business, 09.10.2019 06:50

Mathematics, 09.10.2019 06:50