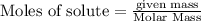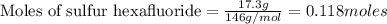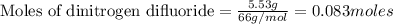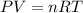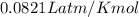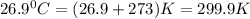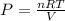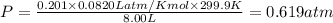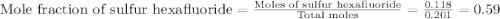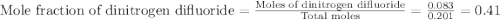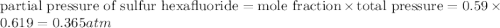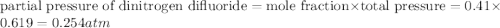Chemistry, 15.04.2020 03:30, briarwilliams9668
A tank at is filled with of chlorine pentafluoride gas and of dinitrogen difluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Be sure your answers have the correct number of significant digits. chlorine pentafluoride mole fraction: partial pressure: dinitrogen difluoride mole fraction: partial pressure: Total pressure in tank:

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
Do you know the correct answer?
A tank at is filled with of chlorine pentafluoride gas and of dinitrogen difluoride gas. You can ass...
Questions in other subjects:

English, 17.12.2019 12:31




History, 17.12.2019 12:31

Mathematics, 17.12.2019 12:31

Mathematics, 17.12.2019 12:31



Biology, 17.12.2019 12:31