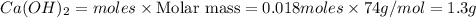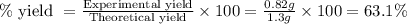Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
...

Chemistry, 15.04.2020 03:36, Crtive5515
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
CaO(s)+H2O(l)→Ca(OH)2(s)
In a particular experiment, a 1.00-g sample of CaO is reacted with excess water and 0.82 g of Ca(OH)2 is recovered. What is the percent yield in this experiment?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Spanish, 06.09.2020 02:01

Biology, 06.09.2020 02:01


Mathematics, 06.09.2020 02:01





Chemistry, 06.09.2020 02:01


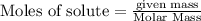
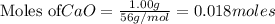
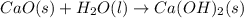
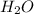 is the excess reagent,
is the excess reagent, 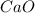 acts as the limiting reagent and it limits the formation of product.
acts as the limiting reagent and it limits the formation of product.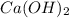
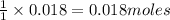 of
of