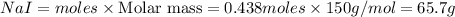Chemistry, 15.04.2020 03:34, alekvtaylor
Iodine is prepared both in the laboratory and commercially by adding Cl2(g) to an aqueous solution containing sodium iodide: 2NaI(aq) +Cl2(g) → I2(s) +2NaCl(aq) How many grams of iodide, NaI, must be used to produce 55.6 g of iodine, I2?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, lilblackbird4
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
Do you know the correct answer?
Iodine is prepared both in the laboratory and commercially by adding Cl2(g) to an aqueous solution c...
Questions in other subjects:

Mathematics, 13.01.2020 06:31

Physics, 13.01.2020 06:31

Chemistry, 13.01.2020 06:31



Physics, 13.01.2020 06:31

Mathematics, 13.01.2020 06:31

Mathematics, 13.01.2020 06:31

Mathematics, 13.01.2020 06:31

History, 13.01.2020 06:31


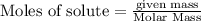

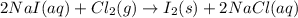
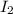 are produced by = 2 moles of
are produced by = 2 moles of 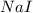
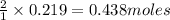 of
of