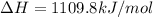Chemistry, 15.04.2020 03:33, tusharchandler124
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combustion = −803 kJ/mol CH4) in the bomb. The temperature changed by 11.1°C. (a) What is the heat capacity of the bomb? kJ/°C (b) A 14.0-g sample of acetaldehyde (CH3CHO) produced a temperature increase of 11.3°C in the same calorimeter. What is the energy of combustion of acetaldehyde (in kJ/mol)?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
Do you know the correct answer?
The heat capacity of a bomb calorimeter was determined by burning 6.91 g of methane (energy of combu...
Questions in other subjects:

History, 28.07.2019 06:30


History, 28.07.2019 06:30


Mathematics, 28.07.2019 06:30


Biology, 28.07.2019 06:30

Chemistry, 28.07.2019 06:30


History, 28.07.2019 06:30




 = enthalpy change = -803 kJ/mol
= enthalpy change = -803 kJ/mol = 6.91 g
= 6.91 g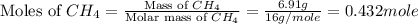



 = change in temperature =
= change in temperature = 


 = 14.0 g
= 14.0 g