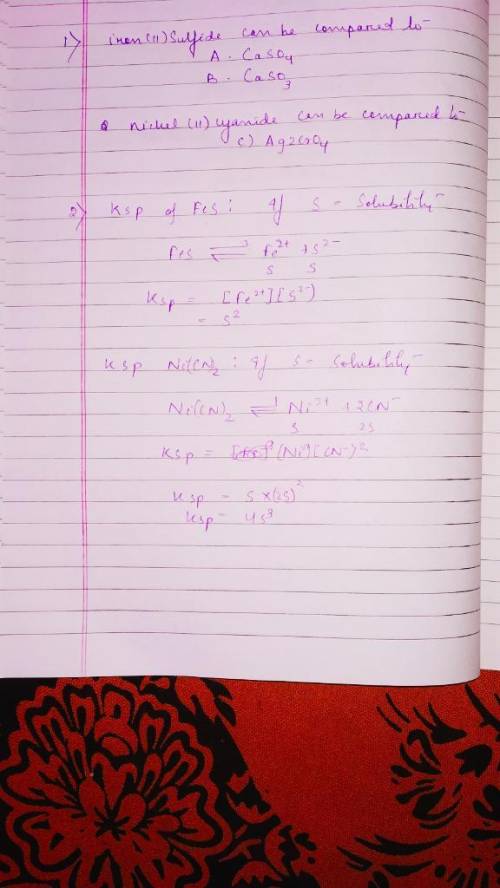Chemistry, 15.04.2020 03:19, patriciahonsakpa6u5f
For each of the salts on the left, match the salts on the right that can be compared directly, using Ksp values, to estimate solubilities. If more than one answer is correct, enter the letters without delimiting characters. 1. lead chloride A. Zn(CN)2 2. zinc carbonate B. Zn3(PO4)2 C. PbSO4 D. Ni(OH)2 Write the expression for Ksp in terms of the solubility, s, for each salt, when dissolved in water. lead chloride zinc carbonate Ksp = Ksp = Note: Multiply out any number and put it first in the Ksp expression. Combine all exponents for s. Submit Answer

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 06:30, angelrenee2000
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent. true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight. a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
Do you know the correct answer?
For each of the salts on the left, match the salts on the right that can be compared directly, using...
Questions in other subjects:


Mathematics, 04.03.2021 18:00


Mathematics, 04.03.2021 18:00

Mathematics, 04.03.2021 18:00

Mathematics, 04.03.2021 18:00

Mathematics, 04.03.2021 18:00


History, 04.03.2021 18:00

English, 04.03.2021 18:00