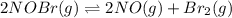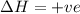Chemistry, 15.04.2020 03:13, joeykyle05
For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. The reaction is endothermic. What do you expect to happen to the concentration of NO if the temperature is doubled? 2NOBr(g) ⇌ 2NO(g) + Br2(g)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 16:00, winnie45
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
Do you know the correct answer?
For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. The reactio...
Questions in other subjects:

Mathematics, 05.05.2020 07:18


Mathematics, 05.05.2020 07:18





History, 05.05.2020 07:18

Geography, 05.05.2020 07:18