
Chemistry, 15.04.2020 03:10, danielburke24
The reaction SO2(g)+2H2S(g)←→3S(s)+2H2O(g) is the basis of a suggested method for removal of SO2 from power-plant stack gases. The standard free energy of each substance are ΔG∘fS(s) = 0 kJ/mol, ΔG∘fH2O(g) = -228.57 kJ/mol, ΔG∘fSO2(g) = -300.4 kJ/mol, ΔG∘fH2S(g) = -33.01 kJ/mol. If PSO2 = PH2S and the vapor pressure of water is 22 torr , calculate the equilibrium SO2 pressure in the system at 298 K.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
Do you know the correct answer?
The reaction SO2(g)+2H2S(g)←→3S(s)+2H2O(g) is the basis of a suggested method for removal of SO2 fro...
Questions in other subjects:

Mathematics, 05.05.2021 17:50

Mathematics, 05.05.2021 17:50






Mathematics, 05.05.2021 17:50


Biology, 05.05.2021 17:50


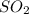 pressure is,
pressure is, 
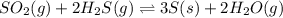
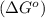 .
.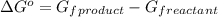
![\Delta G^o=[n_{S(s)}\times \Delta G_f^0_{(S(s))}+n_{H_2O(g)}\times \Delta G_f^0_{(H_2O(g))}]-[n_{SO_2(g)}\times \Delta G_f^0_{(SO_2(g))}+n_{H_2S(g)}\times \Delta G_f^0_{(H_2S(g))}]](/tpl/images/0600/9913/1b30c.png)
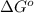 = standard free energy of reaction = ?
= standard free energy of reaction = ?![\Delta G^o=[3mole\times (0kJ/mol)+2mole\times (-228.57kJ/mol)]-[1mole\times (-300.4kJ/mol)+2mole\times (-33.01kJ/mol)]](/tpl/images/0600/9913/f2373.png)


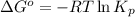
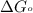 = standard Gibbs free energy = -90.72 kJ/mol
= standard Gibbs free energy = -90.72 kJ/mol
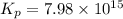

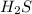 = x
= x





