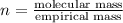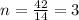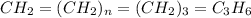Chemistry, 15.04.2020 02:55, bvbbridesmaid5519
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At standard conditions, 112 mL of the gaseous compound weighs 0.21 g. What is the molecular formula for the compound

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, penelopegrace04
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
Do you know the correct answer?
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At st...
Questions in other subjects:



Mathematics, 28.08.2021 21:30





Mathematics, 28.08.2021 21:30

Mathematics, 28.08.2021 21:30


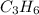
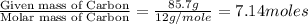
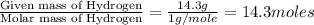
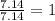
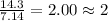
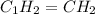
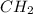 = 1(12) + 2(1) = 14 g/eq.
= 1(12) + 2(1) = 14 g/eq.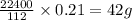 of compound
of compound