
5.00 grams of sodium hydroxide was placed in 100.0 grams of water in a coffee cup calorimeter. The temperature of the water increased from 25.0 deg C to 35.2 deg C. Find the heat of solution (ΔHsoln) for sodium hydroxide in kJ/mol. (Specific heat of water = 4.184J/g degC)?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1


Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
Do you know the correct answer?
5.00 grams of sodium hydroxide was placed in 100.0 grams of water in a coffee cup calorimeter. The t...
Questions in other subjects:


History, 15.07.2019 00:00

Physics, 15.07.2019 00:00

Mathematics, 15.07.2019 00:00



Biology, 15.07.2019 00:00


Mathematics, 15.07.2019 00:00

Mathematics, 15.07.2019 00:00


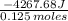
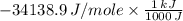 = -34.139 kJ/mol
= -34.139 kJ/mol



