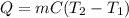In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of HCl are mixed. The HCl had an initial temperature of 44.6 oC and the water was originally at 24.6 oC. After the reaction, the temperature of both substances is 31.3 oC.
a. Was the reaction exothermic or endothermic?Explain.
b. Calculate how much heat the water lost or gained.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
Do you know the correct answer?
In a coffee-cup calorimeter, 100.0 g of H2O and 100.0 mL of HCl are mixed. The HCl had an initial te...
Questions in other subjects:


Mathematics, 25.01.2021 21:00



Mathematics, 25.01.2021 21:00


Computers and Technology, 25.01.2021 21:00