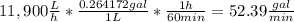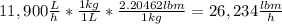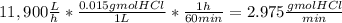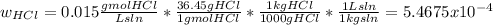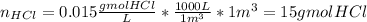The average flowrate of hydrochloric acid solution from a plant is 11,900 Lt/hr. The density is the same as that of water (1kg/Lt). The concentration of HCl in the solution is 0.015gmol/Lt.
Calculate the following:
(i) Flowrate in gallons per minute
(ii) Mass flowrate in lbm / hr
(iii) The number of gram mol of HCl flowing per minute
(iv) The mass fraction of HCl in the solution
(v) The number of lb mols of HCl in 1m3 of solution

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, daigle18383
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2
Do you know the correct answer?
The average flowrate of hydrochloric acid solution from a plant is 11,900 Lt/hr. The density is the...
Questions in other subjects:








Mathematics, 23.05.2020 21:58

Mathematics, 23.05.2020 21:58