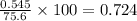Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2
Do you know the correct answer?
Consider the reaction CaCN2 + 3 H2O → CaCO3 + 2 NH3 . This reaction has a 75.6% yield. How many mole...
Questions in other subjects:


Mathematics, 05.08.2021 16:50

Social Studies, 05.08.2021 16:50

Mathematics, 05.08.2021 16:50


Health, 05.08.2021 16:50

Biology, 05.08.2021 16:50

Mathematics, 05.08.2021 16:50

Mathematics, 05.08.2021 16:50

English, 05.08.2021 16:50


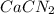 are needed to obtain 18.6 g of
are needed to obtain 18.6 g of 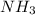
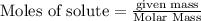
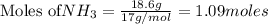
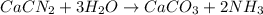
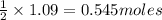 of
of