
Chemistry, 15.04.2020 01:19, taytaycola223
Bohr Model: an electron in a doubly ionized lithium atom +2Li(three protons in the nucleus) makes a transition from the 풏=ퟏto the 풏=ퟑlevel with an associated photon. a)Determine the photon energy associated with this transition. (10pts)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, JKINGblackstar3502
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1


Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 23.06.2019 00:30, ejuarez2020
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
Do you know the correct answer?
Bohr Model: an electron in a doubly ionized lithium atom +2Li(three protons in the nucleus) makes a...
Questions in other subjects:

Mathematics, 25.03.2020 04:48






Social Studies, 25.03.2020 04:48




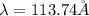
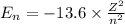
![\Delta E=-13.6\times Z^2[\frac{1}{n_3^2} -\frac{1}{n_1^2}]](/tpl/images/0600/5165/72afd.png)
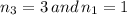
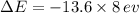
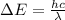
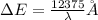
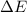 we get,
we get,



